Critical Role of Medical Device Labelling: Ensuring Safety, Compliance, and Patient Well-being
Medical devices play a vital role in diagnosing, treating, and monitoring various medical conditions, enhancing the quality of patient care, and improving outcomes. Yet, an often-overlooked aspect of medical devices takes centre stage in ensuring their safety, regulatory compliance, and effective utilization: Labelling.
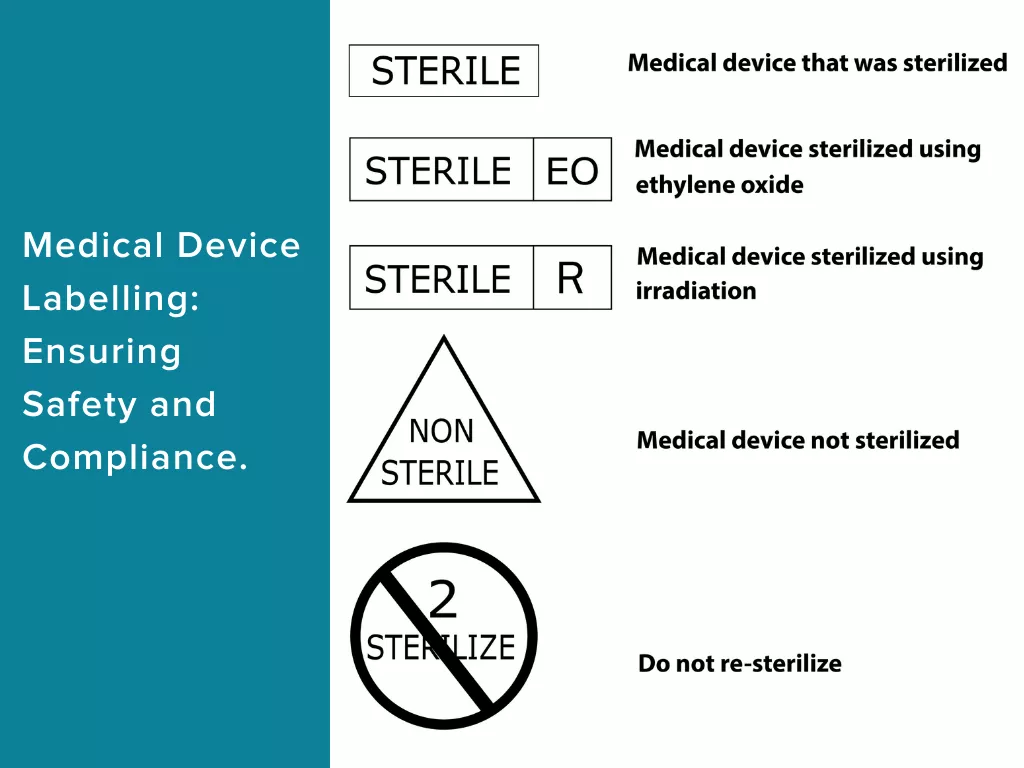
Labelling is more than just a branding tool; it’s a crucial element that healthcare professionals, patients, and regulatory authorities rely on for the safe and informed use of medical devices. This article explores the multifaceted importance of labelling in medical devices and highlights the key factors that make it a pivotal component in the healthcare ecosystem.
1. Ensuring Regulatory Compliance and meeting Legal Obligations
One of the most immediate and critical aspects of labelling in medical devices is its role in ensuring regulatory compliance with regulatory requirements. The medical device industry is highly regulated, with stringent standards and guidelines set by regulatory authorities around the world. In the United States, the Food and Drug Administration (FDA) oversees the approval and marketing of medical devices, while the European Union relies on the Conformity European (CE) marking process. Each country has its regulatory agency with specific requirements.
Labelling acts as a communication bridge between manufacturers and regulators, as it provides a means to convey essential information about the device's intended use, safety precautions, and potential risks. Non-compliance with these regulations can result in serious legal consequences, including recalls, fines, and even criminal charges. Thus, medical device manufacturers must design, review, and update labels regularly to meet evolving regulatory standards.
2. Enhancing Patient Safety and Education
The ultimate goal of medical devices is to improve patient outcomes and safety. Effective labelling plays a pivotal role in achieving this objective by providing critical information to healthcare professionals and patients. Labels must include clear instructions on device usage, handling, storage, and maintenance to prevent errors and ensure patient safety. For example, a label on a surgical instrument must specify the sterilization method to avoid contamination and infection risks.
Patients also benefit from well-designed labels, especially when using home-based medical devices. Take insulin pumps, for instance; these devices are used by individuals with diabetes to manage their blood sugar levels. Clear and concise labelling ensures that patients can use the device safely and effectively, reducing the risk of user errors and adverse events.
Moreover, labels often include information about potential side effects, contraindications, and precautions. This knowledge empowers both healthcare professionals and patients to make informed decisions about device usage, weigh the risks and benefits, and take appropriate actions when necessary. In essence, labelling serves as a powerful tool in patient education, enhancing healthcare literacy and empowering patients in their care journey.
3. Ensuring Traceability and Quality Control
Labelling also plays a crucial role in maintaining traceability throughout the lifecycle of a medical device. Each device must have a unique identifier, such as a serial number or a bar code, on its label. This identifier facilitates tracking and tracing the device from its manufacturing origin to its end-user, helping in quality control and monitoring for any potential issues.
In the event of a product recall or a reported adverse event, the traceability provided by labelling allows manufacturers and regulatory authorities to quickly identify affected devices and take necessary corrective actions. Without proper labelling and traceability, it would be significantly more challenging to manage product recalls and prevent potential harm to patients.
4. Promoting international Standardization and Harmonization
The global reach of the medical device industry necessitates efforts toward international standardization and harmonization. Various countries and regions have their regulatory requirements, making it complex for manufacturers to navigate the global market. Labels must be adapted to meet the specific regulations of each market.
International organizations such as the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) work to develop harmonized standards for medical device labelling. These standards help streamline the labelling process for manufacturers, reduce compliance burdens, and ensure consistency in labelling across different markets. By adhering to international labelling standards, manufacturers can access a broader range of markets and reduce the complexity of compliance management.
5. Differentiating Products and Building Brand Identity
While the primary purpose of labelling in medical devices is to provide essential information and ensure compliance, it also plays a role in product differentiation and brand identity. The visual design and branding elements on a medical device's label can set it apart from competitors and create a recognizable brand identity.
Brand recognition is more than just aesthetics; it influences trust and preference among healthcare professionals and patients. A well-established and reputable brand may instil confidence in the device's safety and effectiveness. However, it's crucial to strike a balance between branding elements and the essential information that must be conveyed to users, ensuring that the label remains clear, informative, and compliant with regulatory requirements.
6. Addressing Accessibility and Multilingual Considerations
In our increasingly globalized world, accessibility and multilingual considerations are becoming more important in medical device labelling. Medical devices are distributed worldwide, and their users come from diverse linguistic backgrounds. Labels must be designed to accommodate this linguistic diversity, ensuring that essential information is accessible to all users, regardless of their language proficiency.
Multilingual labelling not only promotes safety but also aligns with regulatory requirements in many countries. For instance, the European Union often mandates that labelling be available in the official languages of member states. Manufacturers must invest in accurate translation and localization services to ensure that labels are effective in conveying information in different languages.
7. Embracing evolving Technologies and Digital Labels
As technology continues to advance, so too does the potential for innovation in medical device labelling. Digital labels, such as QR codes and Near Field Communication (NFC) tags, are emerging as tools to enhance the user experience and provide access to more extensive information.
Digital labels can link users to online resources, instructional videos, and real-time updates, offering a dynamic and interactive way to engage with device information. This can be especially valuable for complex devices, where traditional printed labels may not provide sufficient space for all necessary information. However, the adoption of digital labels also brings its own set of challenges, including cybersecurity concerns and ensuring user accessibility, particularly for individuals without access to digital devices.
8. Sustainability and Environmental Considerations
In recent years, sustainability has become a key focus across industries, including healthcare. The materials used in medical device labelling, as well as the production and disposal processes, can have significant environmental impacts. Manufacturers are increasingly exploring sustainable labelling options, such as recyclable or biodegradable materials, to reduce their carbon footprint.
Moreover, electronic labelling and digital documentation can reduce the need for printed materials, further minimizing environmental impact. Sustainable labelling practices align with broader corporate social responsibility efforts and reflect a commitment to environmentally friendly healthcare practices.
Conclusion
Labelling is not just a regulatory requirement; it is an integral part of the medical device that supports the safety and effectiveness of these life-saving tools. Manufacturers, regulators, and healthcare professionals need to work together to make sure that labelling continues to improve in a way that benefits patients, healthcare providers, and the industry.
By recognizing and embracing the critical role of labelling, the medical device industry can continue to provide safer, more effective solutions to patients worldwide.
How Decos Can Help Clients
At Decos, we specialize in ensuring medical device labels and IFUs are accurate, compliant, and clear by developing tailored labelling solutions that enhance safety and usability. We help companies meet global regulatory requirements, including FDA, MDR, and ISO standard. Our team mitigates risks by preventing labelling errors, managing the entire lifecycle of labelling documents, and ensuring compliance across international markets. Partnering with us ensures your medical device labels are both compliant and supportive of patient safety.

This blog is written by Kritika Nagane, Systems Engineer (Labeling Content Engineer) at Decos. She is an expert in labeling and regulatory compliance, with experience in developing and updating labels and Instructions for Use (IFUs) for medical devices.
Decos is a cutting-edge technology services partner ready to meet your diverse needs across various industries, including the medical domain. If you have a question about one of our projects or would like advice on your project or a POC, contact Devesh Agarwal. We’d love to get in touch with you!
Discover more
Exploring Degrees of Freedom: From Mechanics to Robotics

Design for Disassembly: A Path to Sustainable Product Lifecycles

